RGX-202: An Investigational, One-Time Gene Therapy Treatment for Duchenne Muscular Dystrophy
REGENXBIO, a global leader in adeno-associated virus (AAV) gene therapy, is currently enrolling participants in the AFFINITY DUCHENNE® study of RGX-202. RGX-202 is an investigational*, one-time gene therapy for the treatment of Duchenne Muscular Dystrophy, also known as DMD or Duchenne.
AFFINITY DUCHENNE® Study
AFFINITY DUCHENNE® is a Phase 1/2/3 study, currently in the Phase 3 (Pivotal) portion, evaluating the safety, tolerability, pharmacodynamics (effect of a medicine on the body), pharmacokinetics (how the body absorbs and metabolizes a medicine) and efficacy of a one-time intravenous (IV) dose of RGX-202 gene therapy in boys with Duchenne.
This is an open-label study, meaning that there is no placebo group. All eligible study participants will receive RGX-202, the investigational gene therapy.
Key Eligibility Criteria*
Your child may be eligible to participate in the AFFINITY DUCHENNE® study if he:
- Is 1 year of age or older
- Has a genetic diagnosis of Duchenne
- Can complete certain ambulatory function tests based on age, including the ability to stand, walk, and move from one place to another
- Tests negative for antibodies to the gene therapy AAV8 vector. Antibodies can prevent the gene therapy from working as intended.^
Study Status
- This study is currently enrolling
Study Evaluations*
The AFFINITY DUCHENNE study team will conduct multiple assessments to evaluate:
- Safety and tolerability
- Efficacy (muscle strength and function) as measured by tests including Time to Stand, Time to Run/Walk, and other assessments based on age
- Microdystrophin protein levels as measured by a muscle biopsy
*This is not a complete list of eligibility criteria and assessments. Criteria may change in the future. For more information about the trial and whether your child may be eligible, talk with your child’s doctor, an AFFINITY DUCHENNE study doctor, or visit the study page (identifier NCT05693142) on ClinicalTrials.gov.
^A separate study, AFFINITY BEYOND®, can test the antibody status of your child to help determine potential eligibility for the AFFINITY DUCHENNE gene therapy study. To learn more, visit the AFFINITY BEYOND study page (identifier NCT05683379) on ClinicalTrials.gov. Results of this study do not guarantee acceptance into AFFINITY DUCHENNE.
What AFFINITY DUCHENNE® Study Participants Can Expect:
All AFFINITY DUCHENNE study participants will receive a single intravenous (IV) dose of RGX-202 gene therapy. During their participation in the study, multiple visits to the study site will be required and families will have many opportunities to interact with the study team. Below is a brief outline of what is involved when participating in the trial:Eligibility Screening
The study team will schedule a screening visit to determine if your child qualifies for the study and review the informed consent form with you in detail.
- If you agree to have your child participate in the study, you’ll sign the informed consent form and begin the screening process
- During screening, certain assessments and tests are performed to determine eligibility. These could include function tests, an MRI, and a muscle biopsy
- The study team will review your child's health and possibly request medical records
- The process for determining eligibility may take up to two (2) months
Initial Contact with Study Team
If you are interested in having your child participate in the AFFINITY DUCHENNE study, you will need to talk with a member of the study team to determine if your child may be eligible. The study team will review the details of the study with you, including what will be required, and start the informed consent process.
- Informed consent is a process by which patients and families receive important information about medical treatment or a clinical trial study, including possible risks and benefits
- The study team will review this information with you to help you decide if you want your child to participate in the trial
Study Participation
If your child qualifies and you decide to enroll him in AFFINITY DUCHENNE, study participation will last two (2) years.
- Dosing (receiving RGX-202 gene therapy): All participants will receive a single intravenous (IV) dose of RGX-202
- Other medications: In addition to receiving RGX-202 gene therapy, participants will be given immune suppression and antibiotic drugs prior to dosing and will continue to receive them for several weeks after being dosed with RGX-202. Immune suppression medications are commonly used at the start of gene therapy. Talk with the study team if you have any questions
- Post-Dosing: You will have multiple visits to the study site to monitor your child’s health and to complete different tests and assessments to see how the therapy may be working
Long-Term Follow Up
At the end of the study, you will be encouraged to enroll your child in a three-year follow-up study at the AFFINITY DUCHENNE study site. Your child will continue to be followed by the study team to understand the long-term safety and efficacy of RGX-202. Site visits during the long-term follow-up will be less frequent than those in the main study.
Study Costs
REGENXBIO, the sponsor of the AFFINITY DUCHENNE Study, will cover reasonable costs of transportation, lodging, and other study-related expenses for participants and their families.
The study team can review additional details with you.
AFFINITY DUCHENNE® Locations
Study sites in the U.S. are now open and enrolling. Additional study site locations may open over time.
Please visit the study page (identifier NCT05693142) on ClinicalTrials.gov for the most up-to-date information about study sites and site contact information.
United States Locations
Arkansas Locations
Little Rock, Arkansas, United States, 72202
Recruiting
Arkansas Children's Hospital
Contact: Amber Evans
Contact: Aravindhan Veerapandiyan, MD
California Locations
Palo Alto, California, United States, 94304
Recruiting
Stanford School of Medicine /Division of Neuromuscular Medicine
Contact:
Contact: Carolina Tesi-Rocha, MD
Colorado Locations
Aurora, Colorado, United States, 80045
Recruiting
Children's Hospital Colorado
Contact: Michele Yang
Contact: Hannah Kleiner
Contact: Michele Yang, MD
Florida Locations
Gainesville, Florida, United States, 32610
Recruiting
University of Florida
Contact: Melissa Lewis
Contact: Barry Byrne, MD
Georgia Locations
Atlanta, Georgia, United States, 30329
Recruiting
Rare Disease Research
Contact: Lily Goggans
Contact: Maureen Ikpeoha
Contact: Han Phan, MD
Illinois Locations
Chicago, Illinois, United States, 60611
Recruiting
Ann & Robert H. Lurie Children's Hospital of Chicago
Contact: Nicole Geanous
Contact: Nancy L Kuntz, MD
Iowa Locations
Iowa City, Iowa, United States, 52242
Recruiting
University of Iowa
Contact: Laura Knosp
Contact: Dimah Saade, MD
Massachusetts Locations
Worcester, Massachusetts, United States, 01608
Recruiting
University of Massachusetts Chan Medical School
Contact: Tyler Mola
Contact: Eleonora D'Ambrosio, MD
Ohio Locations
Cincinnati, Ohio, United States, 45229
Recruiting
Cincinnati Children's
Contact: Angela Edmondson
Contact: Cuixia Tian, MD
Oregon Locations
Portland, Oregon, United States, 97239
Recruiting
Oregon Health & Science University
Contact: Beata Dyar
Contact: Erika Finanger, MD
Texas Locations
Dallas, Texas, United States, 75390
Recruiting
The University of Texas Southwestern Medical Center
Contact: Elaine Most
Contact: Susan Iannaccone, MD
Virginia Locations
Norfolk, Virginia, United States, 23510
Recruiting
Children's Hospital of the King's Daughters
Contact: Erika Paradiso
Contact: Crystal Proud, MD
Richmond, Virginia, United States, 23298
Recruiting
Children's Hospital of Richmond at Virginia Commonwealth University
Contact: Falgun Patel
Contact: Amy Harper, MD
Canada Locations
Alberta Locations
Calgary, Alberta, Canada, T3B 6A
Not yet recruiting
Alberta Children's Hospital
Contact: Israt Yasmeen, MBT
Contact: Jean Mah, MD
British Columbia Locations
Vancouver, British Columbia, Canada, V65 3N1
Recruiting
BC Children's Hospital
Contact: Nela Martic
Contact: Kathryn Selby, MD
Ontario Locations
London, Ontario, Canada,
Recruiting
Children's Hospital London Health Science Centre
Contact: Rhiannon Hicks
Contact: Craig Campbell, MD
Ottawa, Ontario, Canada, K1H 8L1
Recruiting
Children's Hospital of Eastern Ontario
Contact: Laura Thompson
Contact: Hugh McMillan, MD
Scroll to see more locations
Learn More About RGX-202
RGX-202 is an investigational gene therapy given once through an intravenous (IV) infusion. It is designed to use a viral (AAV) vector called NAV® AAV8 to deliver a transgene that provides the instructions for your muscle cells to make a novel microdystrophin protein.
Microdystrophin is a shorter version of dystrophin, which is a naturally occurring protein needed for muscles to work properly. Researchers use microdystrophins in gene therapy because the gene that makes full-length dystrophin is too large to fit into the AAV vector that delivers the treatment.
Once the microdystrophin protein is made, it may protect muscle function.
The CT domain is an important part of the dystrophin protein. In animal studies, the CT domain has been shown to protect the muscle and improve the muscle’s ability to repair itself.
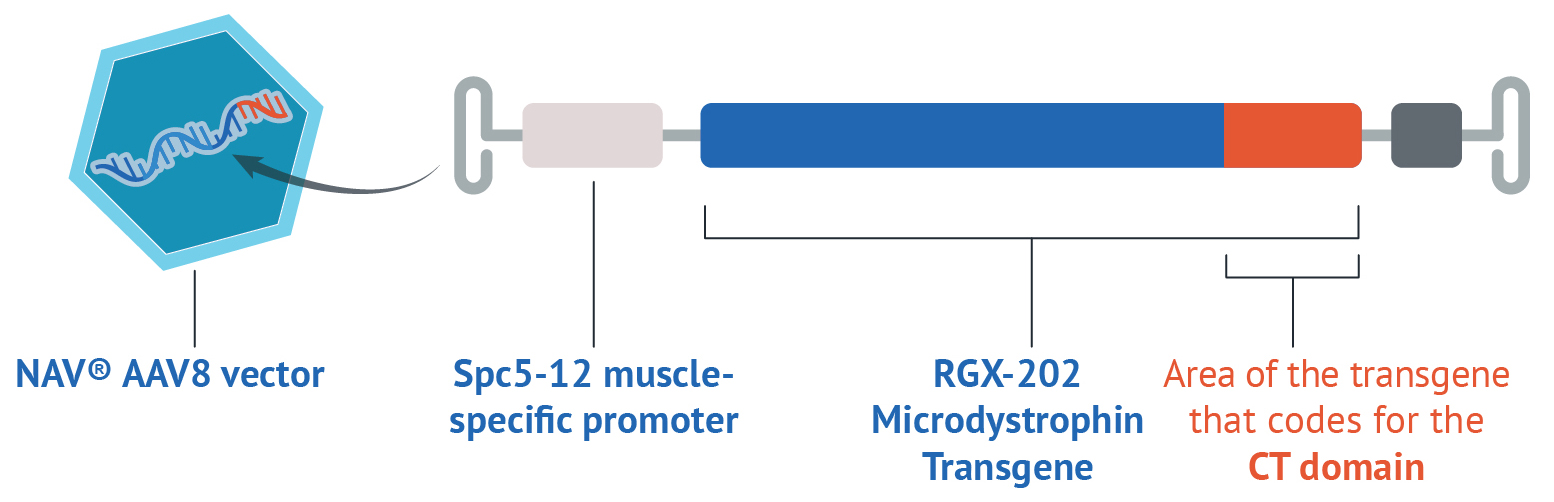
The RGX-202 gene therapy consists of several components designed to work together to deliver RGX-202 to muscle cells and instruct the cells to make the RGX-202 microdystrophin protein.
Vector
Vectors are modified viruses that function as vehicles to deliver therapeutic genetic material, such as a gene, directly into a cell. Vectors are not known to cause disease in humans. RGX-202 uses an adeno-associated viral (AAV) vector called AAV8 because it targets muscle cells.
RGX-202 Microdystrophin Transgene
A transgene is a therapeutic gene that contains instructions for cells, telling the cell how to create a specific protein. The RGX-202 transgene instructs the muscle cells in the body to produce the RGX-202 microdystrophin protein.
Among gene therapies that are approved or in development for the treatment of Duchenne, RGX-202 is the only gene therapy that provides instructions to include the C-terminal (CT) domain, making the RGX-202 microdystrophin protein the closest to the naturally occurring dystrophin.
Promoter
A promoter is essential to controlling the expression of the therapeutic gene. The Spc5-12 promoter for RGX-202 targets muscle cells.
Contact Us
Caregivers and Patients
If you would like to contact a member of the REGENXBIO Patient Advocacy team, please email us at Duchenne@regenxbio.com. For additional information and study site contact information, visit the study page (identifier NCT05693142) on ClinicalTrials.gov